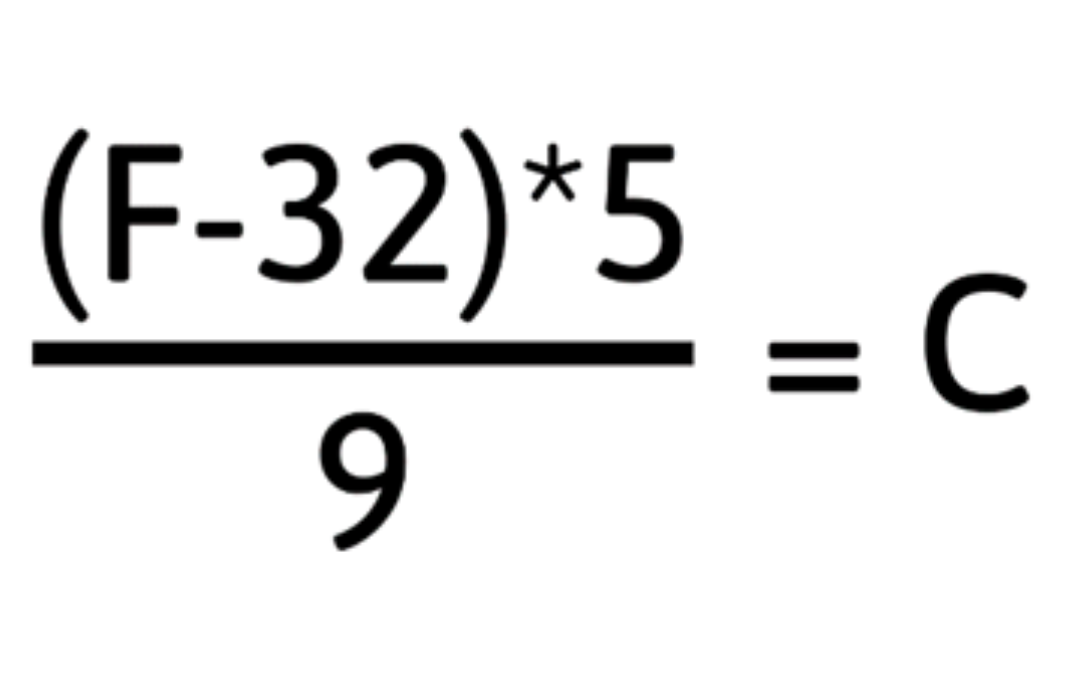What is the difference between heat and temperature?
Heat and Temperature are interconnected terms but do not mean the same thing.
Fundamental Concepts
Heat refers to the amount of energy in an object, measuring the total kinetic and potential energy contained by the molecules in that object. Temperature refers to the intensity of heat, measuring the average kinetic energy of molecules in a substance.
Heat is a form of energy, where temperature is a measure of that energy.
As heat is a form of energy, it is important to remember that it is subject to the principle of conservation under the First Law of Thermodynamics – heat can neither be created or destroyed.
Heat is transferred from one object to another due to differences in temperature, in one of three ways:
Heat is transferred from one object to another due to differences in temperature, in one of three ways:
Conduction – heat is transferred between molecules which are in direct contact with one another, without movement of particles.
Convection – heat transfer takes place due to movement of particles from one place to another.
Radiation – heat is transferred through a medium or vacuum where in space in between is not heated up.
Considerations for Effective Heat Management
Considering where you want the heat to go is an important aspect of specifying your cooling solution.
The most straightforward solution is to dissipate the heat into the local ambient, but this comes with penalties. If you site the unit indoors, you may have to increase the power of the HVAC system of your building. If you site the unit outdoors, the performance of the unit could be affected by high or low ambient temperatures.
The alternative is to have a water-cooled unit and eject the heat into a primary cooling water loop. These are not always available and involve connecting the unit up to two sets of inlets and outlets.
Please refer to the below table for further information on the differences between heat and temperature:
| Heat | Temperature | |
| Property | Heat travels from the hotter object to the cooler object. | Temperature rises when heated and falls when cooled |
| Ability to do work | Yes | No |
| Units | Joules Calories BTU (British Thermal Units) kW (kilowatt) |
Kelvin Celcius Fahrenheit Rankine |
| Device | Calorimeter | Thermometer |
| Symbol | Q | T |
Understanding Temperature Scales
| °C Celcius |
There is a 100-degree division between the freezing and boiling points of water. Water freezes at 0°C and boils at 100°C. Absolute zero is -273.15°C
|
| °F Fahrenheit |
Fahrenheit is used frequently in the United States and parts of the Caribbean. Water freezes at 32°F and boils at 212°F. Absolute zero is -459.67°F
|
| K Kelvin |
The Kelvin Scale was designed to set the zero point of the temperature scale at zero. ° is not used in notation with Kelvin. Water freezes at 273.15K and boils at 373.15K. Kelvin is widely used as a scale in scientific equations due to the direct relation to absolute zero.
|
| °R Rankine |
Rankine is not widely used. Rankine is the absolute zero-based equivalent to the Fahrenheit Scale. Water freezes at 491.67°R and boils at 671.677°R.
|
Fahrenheit and Celsius or Kelvin cannot be interchangeable units when describing temperature due to the difference in degree division, and where absolute zero falls on the scale. To use different units, conversions must be done.
To convert Fahrenheit to Celsius, use the following equation: